南湖新闻网讯(通讯员 郑璇)近日,工学院晏水平教授课题组研究成果以“Glycine-mediated leaching-mineralization cycle for CO2 sequestration and CaCO3 production from coal fly ash: Dual functions of glycine as a proton donor and receptor”为题在Chemical Engineering Journal发表。
研究提出了基于甘氨酸循环的粉煤灰矿化CO2制备球霰石型CaCO3的新机制,系统阐释了氨基酸对碱性固废中碱土金属离子浸提、CO2高效吸收和球霰石型碳酸钙定向调控的三重增效机制,利用氨基酸得失质子及络合特性,通过创新性的反应路径设计和精确的反应条件控制,实现粉煤灰高效浸出-CO2矿化、CaCO3晶型形貌定向调控、以及氨基酸原位再生,为碱性固废增值化利用提供了新思路,助力固废资源化及“双碳目标”的达成。
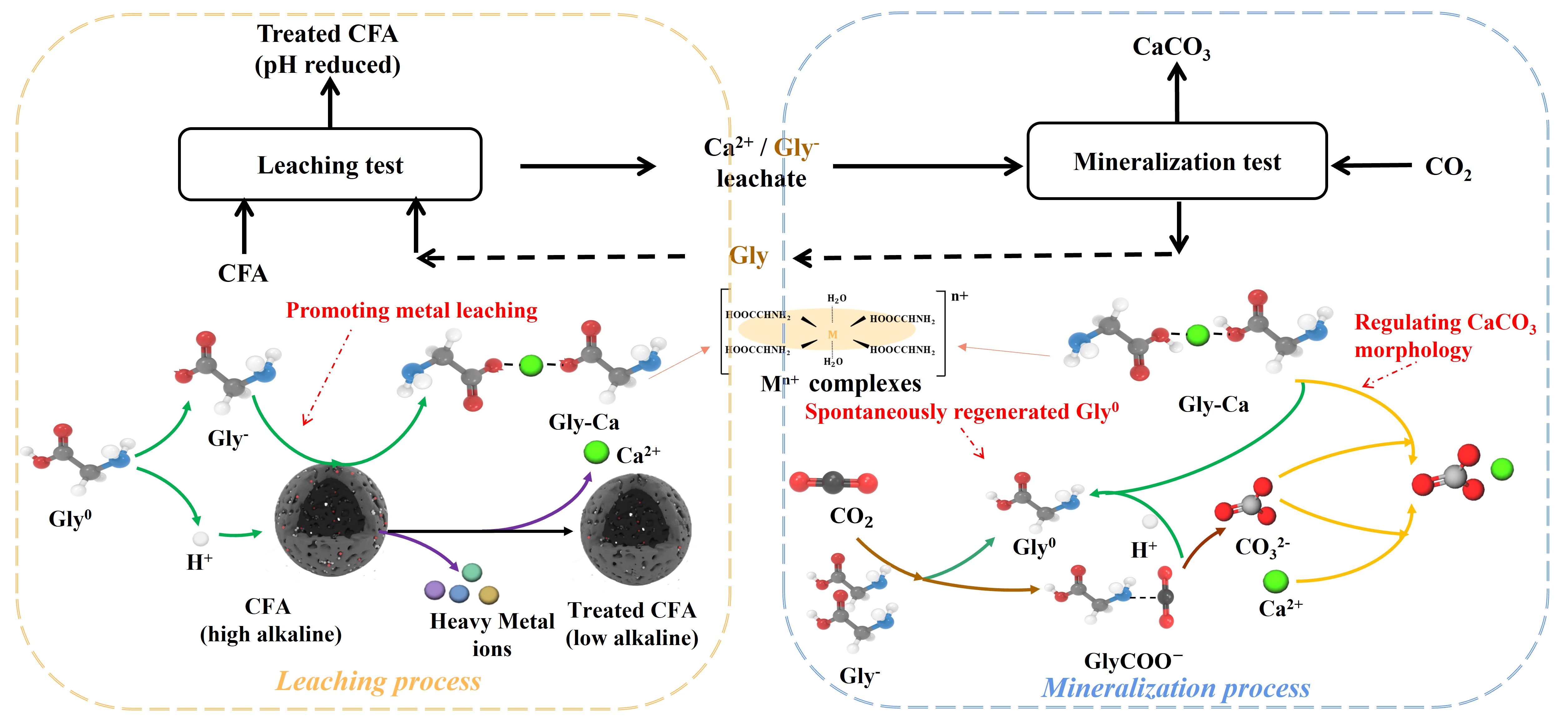
基于甘氨酸循环的粉煤灰浸提-CO2矿化(LMC)新机制示意图
基于碱性固废的CO2矿化技术是一种减废降碳协同增效技术,不仅可降低电厂碳排放,还可促进粉煤灰资源化利用。现有直接法粉煤灰CO2矿化工艺虽然过程相对简单、处理量更大,但是存在反应速率低和产品价值低等问题。间接法CO2矿化工艺过程相对复杂,能联产高附加值的高纯CaCO3产品,具有更好的发展前景。但间接法矿化工艺存在浸出效率较低、矿化过程CaCO3产率和CO2去除率较低、浸出剂难以再生、浸出剂循环性能衰减过快等问题有待解决。
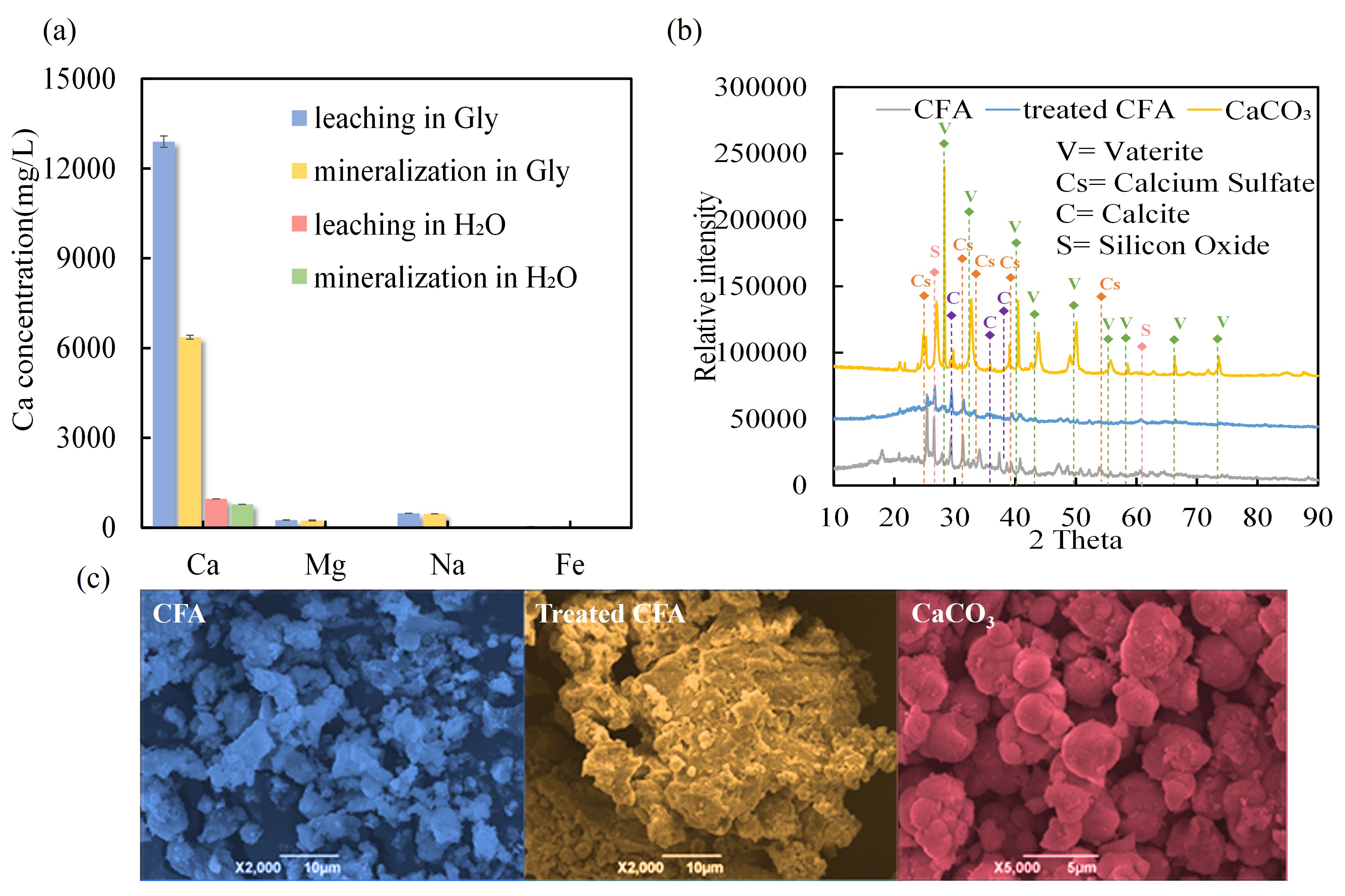
基于甘氨酸循环的粉煤灰浸提-CO2矿化(LMC)可行性探究
针对上述问题,研究提出利用可循环的甘氨酸溶液为浸提剂,在温和条件下对高钙粉煤灰中的钙基组分进行高效浸提,形成的浸提液作为一种良好的CO2吸收剂,可实现CO2脱除并制备高纯球霰石型CaCO3,同时还实现甘氨酸的原位再生。
研究表明,在温和的操作条件下,利用甘氨酸溶液,粉煤灰中Ca2+浸出率为42%,CaCO3产率为89 g/kg-粉煤灰。X射线衍射和扫描电镜分析表明,CaCO3产品的矿物相以球霰石为主。机理探讨表明,甘氨酸在该工艺中起着多重作用:(1)在浸提过程中,甘氨酸起到质子供体和螯合剂的作用,促进了碱土金属离子的浸出,提高了碱性矿物相的浸提容量;(2)在矿化过程中,甘氨酸起到质子受体的作用,利用其氨基官能团对CO2的亲和性提升CO2吸收速率,利用羧基官能团对CaCO3生长的调控特性定向诱导球霰石型CaCO3的形成;(3)在多重浸提-矿化循环过程,利用其两性离子可得失溶液质子的特性提供pH缓冲性能,从而实现反应过程效率的提升。五次浸出-矿化循环的试验结果表明,氨甘氨酸溶液的浸出能力、CaCO3产量和甘氨酸损失相近,验证了该工艺的稳定性。
我校工学院博士生郑璇为论文的第一作者,纪龙副教授和晏水平教授为论文共同通讯作者。该研究得到了国家自然科学基金、湖北省自然科学基金、中央高校基本业务经费等项目资助。
审核人:晏水平
【英文摘要】
The state-of-the-art CO2 mineralization technologies involved Ca/Mg leaching and ensued mineralization still struggle with the slow reaction kinetics and frequent pH swings assisted by exogenous chemicals consumption (i.e., low pH for Ca/Mg leaching but high pH for CO2 dissolution and solid carbonates precipitation). This study proposed a glycine-mediated leaching-mineralization cycle (LMC) process, which can simultaneously achieve promising Ca/Mg leaching efficiency, high mineral carbonation efficiency, and production of high-purity CaCO3 from coal fly ash (CFA) at mild operating conditions in an in-situ recyclable amino acid solution. The technical feasibility of the process was initially investigated in individual leaching and mineral carbonation experiments in glycine (Gly0) solutions using a typical CFA. A Ca2+ leaching efficiency of 42.17% and a CaCO3 yield of 89.10 g/kg were achieved in the Gly0 solution. Mineralogy and morphology analysis revealed that the CaCO3 obtained after the carbonation reaction was mainly present as vaterite. The mechanism exploration revealed that Gly-species acted as a proton donor and chelating agent in the leaching step which enhanced the Ca2+ leaching, a proton receptor in mineralization step which accelerated CO2 mass transfer, and a crystal regulator in carbonates precipitation. In addition, the cyclic performance of the LMC process was investigated in multicycle leaching-carbonation experiments. Results showed that the leaching capacities, CaCO3 yield, and Gly0 loss were similar in five cycles of LMC experiments, verifying that the process is stable.
论文链接:https://www.sciencedirect.com/science/article/pii/S1385894722013985

