南湖新闻网讯(通讯员 郑倩)近日,我校资源与环境学院土壤化学与环境团队联合环境污染与修复团队在非稳态活性组分与砷界面反应行为及其环境效应方面取得系列进展,相关研究成果分别以“Micropore sites in ferrihydrite are responsible for its higher affinity towards As(III) relative to As(V)”“Insights into the underlying mechanisms of stability working for As(III) removal by Fe-Mn binary oxide as a highly efficient adsorbent”为题发表在期刊Geochimica et Cosmochimica Acta和Water Research上。
变价非(亚)稳态铁(氢)氧化物广泛存在于土壤和沉积物中的活性组分,且种类丰富。然而,不同类型的铁(氢)氧化物微观结构具有明显差异,这也使得它们表现出独特的反应性,从而显著影响污染物的迁移和命运,特别是致癌元素砷。其中,水铁矿作为一种弱结晶非稳态的铁氢氧化物,对控制砷的命运具有重要贡献。学术界普遍认为As(III)的毒性比As(V)大,且对铁(氢)氧化物的亲和力更弱。但是,有研究发现水铁矿的As(III)饱和吸附容量远远大于As(V)。尽管人们对非稳态水铁矿结构和其表面砷吸附行为进行了大量研究,目前还不清楚为什么As(III)比As(V)对水铁矿表面具有较高的吸附亲和力。
基于此,团队成员借助正电子湮灭寿命光谱(PAL)表征技术,首次证实了非稳态水铁矿表面存在丰富的原子缺陷簇(V10-20), 因而形成了“缺陷簇”类微孔结构。这些丰富的“缺陷簇”类微孔结构对增加As(III)吸附容量起着至关重要的作用,主要归因于As(III)较小的水合离子半径,更容易利用缺陷簇类微孔位点(图1)。相关研究对于重新认识不同形态砷在污染土壤和地下水中的迁移风险和命运具有重要意义。
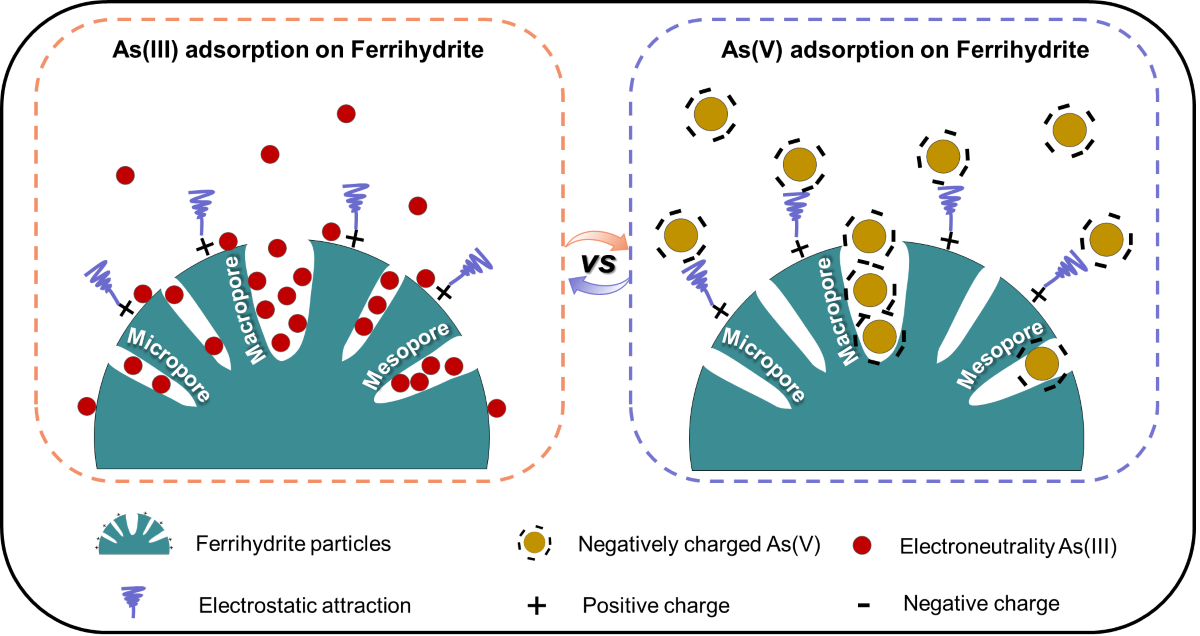
图1 水铁矿对As(III)和As (V)亲和性显著差异的潜在机制
此外,团队针对环境中二元变价非稳态铁和锰活性组分与As(III)之间发生的复杂的界面反应(如吸附、催化、氧化)如何影响其自身的稳定问题,利用自行组装的多组分界面反应装置,探究了活性组分铁锰二元氧化物吸附As(III)的耦合界面反应过程与自稳定机制。研究发现,铁和锰二元氧化物五次吸附/氧化As(III)后仍具有良好的稳定性。As(III)的氧化/吸附行为与铁锰二元体系释放低价Fe、Mn离子相耦合,释放到溶液中的Fe(II)和Mn(II)与铁锰氧化物通过吸附、氧化、催化等过程,逐步生成新的铁氧化物和锰氧化物。从而减少了Fe(II)和Mn(II)释放造成的矿物损失,确保了二元非稳态组分铁锰与As(III)互作过程的自稳定(图2),相关研究从微观机理层面揭示了非稳态铁和锰二元组分在处理实际砷污染土壤和地下水中具有长效稳定性,为后续开发长效、低成本砷污染修复材料提供了理论支撑。
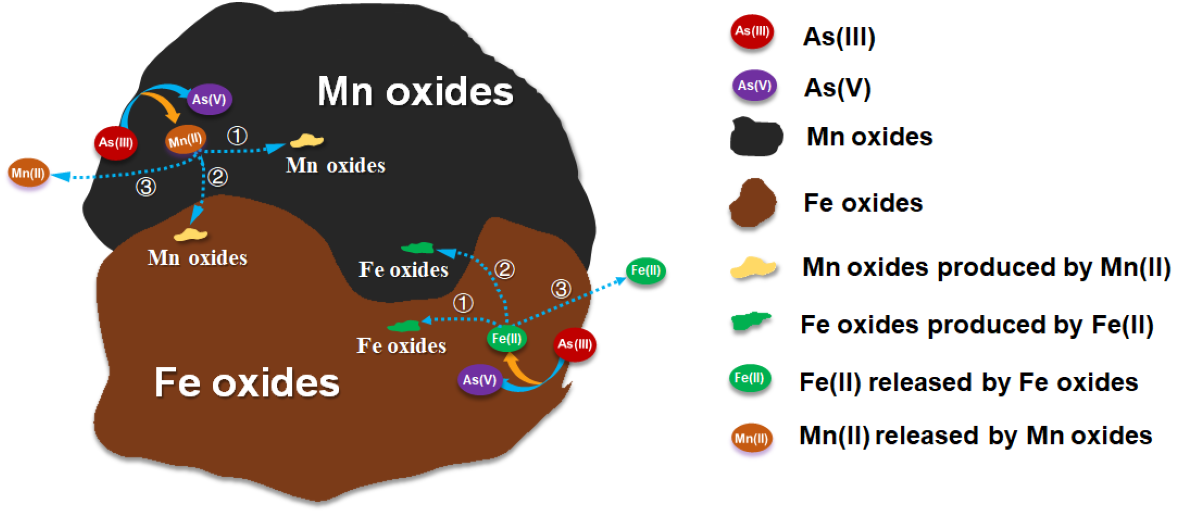
图2 铁锰二元氧化物吸附As(III)的耦合界面反应过程与自稳定机制
系列论文第一作者为我校资源与环境学院博士生郑倩,侯静涛副研究员为论文通讯作者。我校涂书新教授、谭文峰教授、汪明霞教授、熊双莲副教授、曹梦华副教授和熊娟副教授,中国科学技术大学张宏俊研究员和叶邦角教授,以及德国图宾根大学Andreas Kappler教授等参与指导了相关研究工作。相关研究得到国家自然科学基金,武汉市知识创新专项曙光计划和中央高校基本科研业务费的资助。
审核人 侯静涛
【英文摘要1】
Poorly-crystalline ferrihydrite is ubiquitous in the environment and has important contributions to controlling the fate of arsenic in sediments and soils. Although there is evidence that ferrihydrite has a higher affinity towards As(III) relative to As(V), little is known about how and why As(III) is readily immobilized by ferrihydrite. In this study, ferrihydrite was employed to evaluate the As(III) and As(V) adsorption behavior. The properties of ferrihydrite such as morphology, pore size distribution, arsenic adsorption species, and adsorption energies of arsenic at different sites were carefully examined using TEM-EDS mapping, positron annihilation lifetime (PAL) spectroscopy, X-ray absorption spectroscopy (XAS), N2 adsorption isotherms, and theoretical calculations of density function theory (DFT). Batch adsorption experiments revealed that the maximum adsorption of As(III) (839.7 μmol g-1) on ferrihydrite, fitted by Langmuir model, was considerably larger than that of As(V) ( 372.4 μmol g-1). PAL characterization and pore size distribution analysis demonstrated that ferrihydrite had an abundance of vacancy cluster-like micropores, which consisted of 10-20 atom deficiencies (V10-20). The greater As(III) adsorption to ferrihydrite was attributed to its surface adsorption sites as well as its abundant micropore adsorption sites, available only to As(III), due to its size matching well with ferrihydrite micropores, thus significantly contributing to greater As(III) immobilization.
【英文摘要2】
Fe-Mn binary oxide has received increasing interest in treating As(III)-containing polluted groundwater due to its low cost and environmental friendliness. Although the stability of Fe-Mn binary oxide is as important as its adsorption ability, little is known about whether and why Fe-Mn binary oxide is stable during As(III) removal. In this study, five successive cycles were conducted to evaluate the stability of Fe-Mn binary oxide for As(III) removal. As(III) oxidation/adsorption kinetics and the speciation distribution of the released Fe and Mn elements within single Fe oxide, Mn oxide, and Fe-Mn binary oxide were investigated by using characterization techniques of TEM-EDS mapping, selected area electron diffraction (SAED), and XPS combined with a binary component reactor, where Fe and Mn oxides were separated by a semipermeable membrane. The results revealed that Fe-Mn binary oxide could maintain excellent stability, although As(III) oxidation/adsorption behavior was coupled with the release of Fe and Mn ions from its surface. The great stability of Fe-Mn binary oxide for As(III) removal was attributed to the rapid return of aqueous Fe(II) and Mn(II) to the solid surface, which subsequently formed new mineral phases mediated by Fe and Mn oxides, thus considerably decreasing the loss of released Mn(II) and Fe(II).
论文链接:
