南湖新闻网讯(通讯员 张亚格)近日,我校动物科学技术学院、动物医学院崔旻教授团队在Journal of Neuroinflammation杂志上发表了题为“Type I/type III IFN and related factors regulate JEV infection and BBB endothelial integrity”的研究论文,阐释了I型/III型干扰素及相关因子在JEV感染和血脑屏障调控中的作用,为寻找日本脑炎治疗药物靶点提供了新的线索。
日本脑炎病毒(JEV)感染导致日本脑炎(JE)。该病毒感染通常伴随着血脑屏障 (BBB) 完整性的破坏和中枢神经系统 (CNS) 炎症,但发病机制尚未完全解析。作为BBB的主要组成细胞,脑微血管内皮细胞(Brain microvascular endothelial cells, BMECs)分隔了血液循环和脑实质,维持着 CNS 内环境的稳态。关于 JE 的研究多聚焦于 CNS 炎症因子对 BBB 的破坏,而JEV 感染引起的 BMECs细胞的相关变化知之甚少。实际上,JEV在脑微血管内皮细胞(BMEC)中建立感染被认为是病毒穿透血脑屏障的关键初始事件。因此,团队基于转录组测序数据,比较全面地对 JEV 诱导的 hBMECs 细胞内生物信号转导进行了阐述,为天然免疫因子与 BBB 之间的相互调控提供新的理论基础,总结图如下:
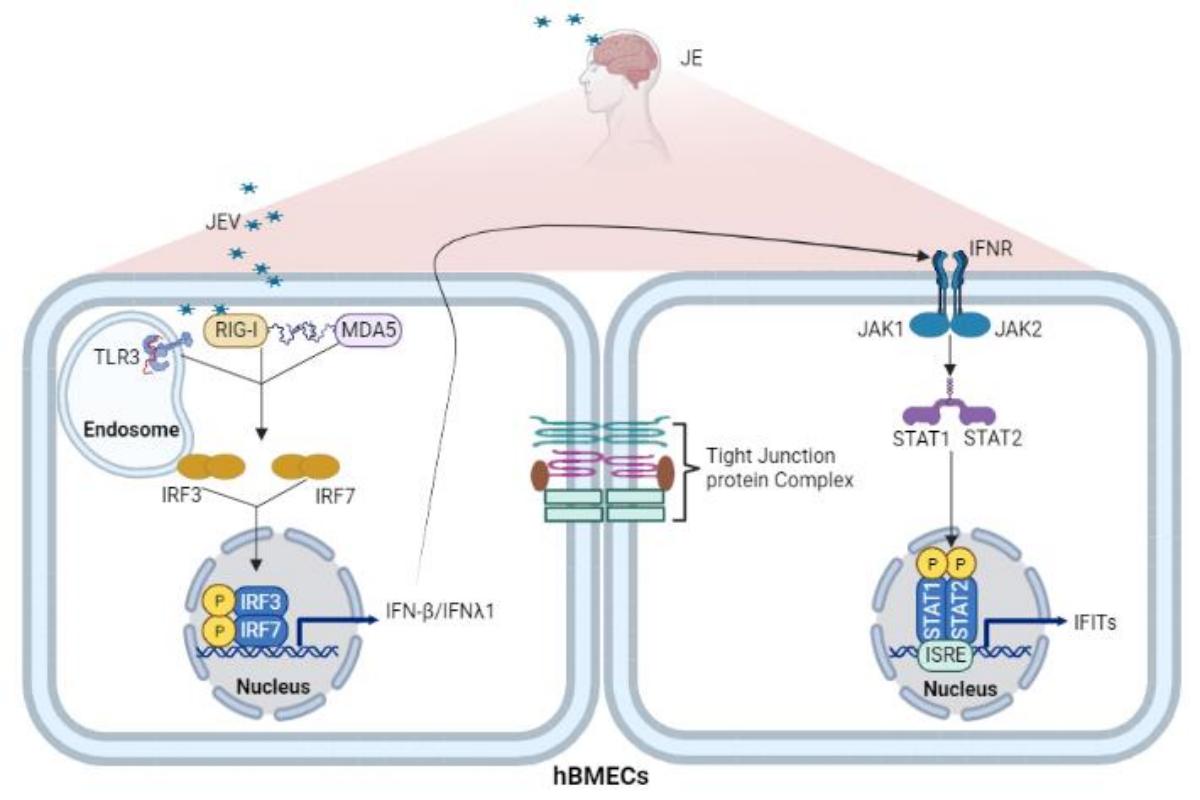
III型干扰素及相关因子在JEV感染和血脑屏障调控中的作用(1)
首先,作者探究了JEV感染人源脑微血管内皮细胞(hBMEC)所引起的细胞特征性变化。结果提示 JEV 感染并未导致 hBMECs 细胞发生病变但在感染后期影响了细胞活性。随后,为了充分阐明 JEV 和 hBMECs 细胞之间的相互作用,对JEV 感染后不同时间点的hBMECs 细胞进行了转录组测序。通过一系列分析,发现感染后的一系列差异表达基因主要富集在宿主细胞的天然免疫应答和内皮细胞通透性调节等通路。进一步的研究表明,模式识别受体(pattern-recognition receptors,PRR,包括 TLR3、RIG-I 和 MDA5)通过识别 JEV 并启动 IRF/IFN 信号传导。 IFN 通过 JAK/STAT 途径触发具有四肽重复序列 (IFIT) 的干扰素诱导蛋白的表达。而在屏障稳态的调控中,不同的 PRRs 发挥了迥异的功能。外源的IFN(IFN-β和IFN-λ1)可以显著减轻内皮屏障的损伤。尽管IFITs与IFN之间存在了复杂的调控,但IFITs并不是IFN稳定内皮屏障所必须的。
我校动物科学技术学院、动物医学院博士研究生张亚格为论文第一作者,崔旻为论文通讯作者。该研究得到国家自然科学基金、国家重点研发计划项目等项目资助。
Abstract
Background: Japanese encephalitis virus (JEV) remains a predominant cause of Japanese encephalitis (JE) globally. Its infection is usually accompanied by disrupted blood‒brain barrier (BBB) integrity and central nervous system (CNS) inflammation in a poorly understood pathogenesis. Productive JEV infection in brain microvascular endothelial cells (BMECs) is considered the initial event of the virus in penetrating the BBB. Type I/III IFN and related factors have been described as negative regulators in CNS inflammation, whereas their role in JE remains ambiguous.
Methods: RNA-sequencing profiling (RNA-seq), real-time quantitative PCR, enzyme-linked immunosorbent assay, and Western blotting analysis were performed to analyze the gene and protein expression changes between mock- and JEV-infected hBMECs. Bioinformatic tools were used to cluster altered signaling pathway members during JEV infection. The shRNA-mediated immune factor-knockdown hBMECs and the in vitro transwell BBB model were utilized to explore the interrelation between immune factors, as well as between immune factors and BBB endothelial integrity.
Results: RNA-Seq data of JEV-infected hBMECs identified 417, 1256, and 2748 differentially expressed genes (DEGs) at 12, 36, and 72 h post-infection (hpi), respectively. The altered genes clustered into distinct pathways in gene ontology (GO) terms and KEGG pathway enrichment analysis, including host antiviral immune defense and endothelial cell leakage. Further investigation revealed that pattern-recognition receptors (PRRs, including TLR3, RIG-I, and MDA5) sensed JEV and initiated IRF/IFN signaling. IFNs triggered the expression of interferon-induced proteins with tetratricopeptide repeats (IFITs) via the JAK/STAT pathway. Distinct PRRs exert different functions in barrier homeostasis, while treatment with IFN (IFN-β and IFN-λ1) in hBMECs stabilizes the endothelial barrier by alleviating exogenous destruction. Despite the complex interrelationship, IFITs are considered nonessential in the IFN-mediated maintenance of hBMEC barrier integrity.
Conclusions: This research provided the first comprehensive description of the molecular mechanisms of host‒pathogen interplay in hBMECs responding to JEV invasion, in which type I/III IFN and related factors strongly correlated with regulating the hBMEC barrier and restricting JEV infection. This might help with developing an attractive therapeutic strategy in JE.
Keywords: Human brain microvascular endothelial cells; IFITs; IFNs; Japanese encephalitis virus; PRRs; RNA-Seq.
审核人 崔旻
